This article was released as Pharm Edaily Premium Content on October 28, 2025, at 8:55 AM.
[Kim Jiwan, Edaily Reporter] On October 27, Korean biotech firms unveiled a string of major developments from global conference invitations and verified drug efficacy to bold demonstrations of responsible leadership.
 |
D&D Pharmatech earned international recognition after its Phase 2 results for a fatty liver treatment were selected for the prestigious “Late-Breaking Abstract” session at the American Association for the Study of Liver Diseases (AASLD).
Intron Biotechnology accelerated its anti-infective program with data showing its novel therapy eradicated 99.9% of Staphylococcus aureus in animal nasal models.
CorestemChemOn, despite a Phase 3 top-line failure for its ALS stem-cell therapy Neuronata-R, drew attention as CEO Yang Gil-an announced a \1.7 billion personal stock purchase a move widely seen as a strong signal of confidence and commitment.
D&D Pharm, “New drug proves efficacy in reducing fatty liver”
D&D Pharmatech’s candidate DD01 has shown significant efficacy in reducing hepatic fat, driving a surge in the company’s shares. The Phase 2 trial results were selected for an oral presentation at AASLD’s Late-Breaking Abstract session an honor reserved for late-stage clinical studies that demonstrate both innovation and data integrity. It is exceptionally rare for a Korean company to be featured in this category.According to KG Zeroin MP Doctor, Curient’s stock jumped 14.79% to close at \193,300.
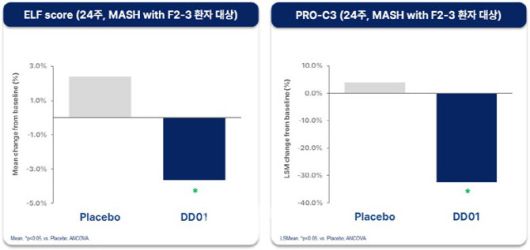 |
Comparison of fibrosis-related biomarker (ELF Score) between the DD01 24-week treatment group and the control group (Source: D&D Pharmatech) |
<이미지를 클릭하시면 크게 보실 수 있습니다> |
MASH (Metabolic dysfunction-associated steatohepatitis) is a progressive liver disease caused by excessive fat accumulation and inflammation due to obesity, diabetes, and dyslipidemia. It can silently advance from fibrosis to cirrhosis and ultimately liver cancer. As there are currently no approved treatments, MASH remains a major unmet medical need worldwide.
DD01 is a dual agonist targeting both GLP-1 (glucagon-like peptide-1) and the glucagon receptor. GLP-1 lowers blood glucose and suppresses appetite, underpinning the success of today’s GLP-1-based obesity drugs, while glucagon receptor activation promotes hepatic fat oxidation and energy expenditure.
The 48-week Phase 2 trial is underway in the United States for patients with MASLD/MASH. Interim 12-week data already demonstrated rapid fat reduction and achievement of primary endpoints related to liver function. The outstanding data earned DD01 a spot at AASLD’s Late-Breaking session for oral presentation by Dr. Mazen Noureddin of Houston Methodist Hospital, a global authority in MASH research who has participated in over 50 major clinical trials, including Madrigal’s Resmetirom, 89bio’s Pegozafermin, and Akero’s Efruxifermin.
CEO Lee Seul-gi stated, “Being invited to AASLD’s Late-Breaking session reflects the global recognition of DD01’s scientific excellence and clinical robustness. With Dr. Noureddin’s presentation, we expect DD01’s technological value to be firmly established in the global market.” She added, “We plan to accelerate partnership discussions and further clinical progress to strengthen our competitiveness in MASH therapeutics.”
Intron, “99.9% elimination of nasal bacteria”
Intron Biotechnology’s novel drug SAL200 (INT-214) successfully eradicated nearly all Staphylococcus aureus colonies residing in the nasal cavity. The company aims to fast-track the development of a nasal decolonization agent to prevent infections in pre-surgical patients.
On the same day, Intron Biotechnology closed at \3,750, up 8.7%.
 |
<이미지를 클릭하시면 크게 보실 수 있습니다> |
Staphylococcus aureus is commonly found in the nose and on the skin of healthy individuals, but when immune defenses are weakened or wounds are present, it can cause life-threatening infections such as sepsis, pneumonia, or endocarditis especially dangerous for surgical patients as a key source of secondary infections.
Intron conducted a proof-of-concept study with an external research partner using cotton rats, whose nasal structures closely resemble humans. Various concentrations of SAL200 were administered after bacterial colonization. The results showed a clear, dose-dependent reduction in bacteria: even at the lowest dose, bacterial counts fell by 98.9%, while higher doses achieved complete sterilization.
Notably, the swab-based intranasal application, the intended clinical delivery method, achieved a 99.9% eradication rate.
Based on these findings, the company plans to finalize the optimal dosage and administration protocol and file for IND approval within this year.
Dr. Jeon Su-yeon, Head of the Biotechnology Research Division, said, “We have confirmed both the powerful decolonization effect and clinical applicability of SAL200. We’re preparing data for IND submission by year-end.”
CEO Yoon Kyung-won added, “SAL200 has already proven therapeutic efficacy in endocarditis models. This study further demonstrates its broad-spectrum antibacterial potential, offering a novel alternative for preventing S. aureus infections and addressing antimicrobial resistance (AMR).”
Corestem, “Leadership accountability through personal stock purchase”
Despite the disappointing top-line results from the Phase 3 trial of Neuronata-R, CorestemChemOn CEO Yang Gil-an announced plans to personally purchase \1.7 billion worth of company stock, reinforcing his long-term confidence in the company’s growth trajectory. The stock rose 7.38% to close at \1,804.
 |
Yang stated, “After confirming the scientific and regulatory potential of Neuronata-R’s global Phase 3 data, I am even more convinced of the company’s long-term value. This purchase reflects not just investment intent, but a strong sense of accountability and faith in our corporate vision.”
Neuronata-R is the world’s first commercially administered stem-cell therapy for amyotrophic lateral sclerosis (ALS), using autologous bone-marrow-derived mesenchymal stem cells (MSCs) injected intrathecally to reduce neuroinflammation and support motor neuron survival. The therapy poses minimal immune rejection risk and has shown sustained safety even with repeated administrations.
Approved conditionally by Korea’s MFDS in 2014, Neuronata-R has been commercially administered to over 400 ALS patients in real-world settings. Leveraging a decade of clinical experience, CorestemChemOn is now conducting a multinational randomized Phase 3 trial (ALSUMMIT, NCT04745299) spanning the U.S. and Korea, while preparing for global regulatory submissions.
Although the overall trial failed to reach statistical significance, subgroup analyses revealed meaningful improvements in motor function (ALSFRS-R) and survival among slow-progressing patients. The company is therefore pursuing FDA accelerated approval pathways.
A company spokesperson noted, “This initiative is not a short-term stock-boosting move, but part of a broader strategy to reinforce corporate competitiveness and restore investor trust through responsible, long-term management.”
이 기사의 카테고리는 언론사의 분류를 따릅니다.
기사가 속한 카테고리는 언론사가 분류합니다.
언론사는 한 기사를 두 개 이상의 카테고리로 분류할 수 있습니다.
언론사는 한 기사를 두 개 이상의 카테고리로 분류할 수 있습니다.


