 |
<이미지를 클릭하시면 크게 보실 수 있습니다> |
Korea’s pharmaceutical industry displayed its changed global status coupled with main track presentations by the sector’s top players in San Francisco this week where some 9,000 executives from 450 healthcare companies came together for the 38th J.P. Morgan Healthcare Conference.
The event, the world’s largest investor show for the pharmaceutical and biotech industry, has drawn market attention since Yuhan Corporation’s $785 million license deal with Gilead inked there a year ago.
Celltrion and Samsung BioLogics, the country’s two leading biosimilar manufacturers, took to the main track podium to pitch their companies.
Samsung BioLogics, the world's top contract manufacturing organization, has been invited to make a presentation at the event’s largest conference venue, where chief executive Kim Tae-han would share the company’s mid- and long-term goals under the title of “Innovation and Growth of Samsung in Biologics Industry.”
Celltrion Chairman Seo Jung-jin, another main track speaker from Korea, introduced the company’s pipeline and future plan and promoted the company’s technology potential by pitching Remsima SC, the world’s first subcutaneous formulation of infliximab.
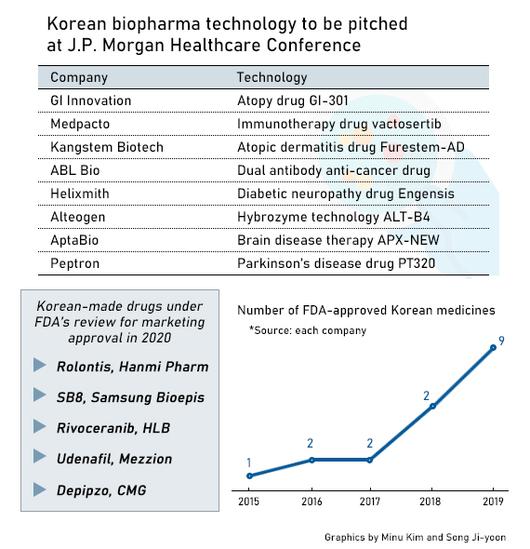
Five other Korean pharmaceutical and biotech firms – Hanmi Pharm, Daewoong Pharm, LG Chem, Hugel and Genexine joined the conference’s Emerging Market Track dedicated to Asian pharmas.
Hanmi Pharm President Kwon Se-chang elaborated on ongoing clinical trials of drugs formulated with the company’s LAPSCOVERY, a platform technology that prolongs the duration of action of biologics and makes it possible for once-weekly or monthly drug administration.
Daewoong Pharm CEO Jeon Seung-ho explained the company’s global clinical development program for gastroesophageal reflux disease drug Fexuprazan, ongoing partnership with Britain-based Avacta Group for immunotherapy development and next steps for Nabota, Korea’s first botulinum toxin product approved both in North America and Europe.
Hugel CEO Son Ji-hoon shared the company’s success factors to become a leading player in the Korean botulinum toxin market, and future plans to leapfrog as a global aesthetic medical company.
JW Pharma met investors to pitch its pipeline of candidate therapeutics including atopic dermatitis drug JW1601 and gout drug URC102. The company is looking for a partner to co-develop JW1601 also to treat macular degeneration and allergic conjunctivitis.
Voronoi, a Korean biotech startup, promoted its five low-molecular compound drugs, including an investigational targeted drug to treat non-small cell lung cancer. The company has already arranged meetings there with 26 global pharmaceutical companies.
[ⓒ Maeil Business Newspaper & mk.co.kr, All rights reserved]
이 기사의 카테고리는 언론사의 분류를 따릅니다.
기사가 속한 카테고리는 언론사가 분류합니다.
언론사는 한 기사를 두 개 이상의 카테고리로 분류할 수 있습니다.
언론사는 한 기사를 두 개 이상의 카테고리로 분류할 수 있습니다.


